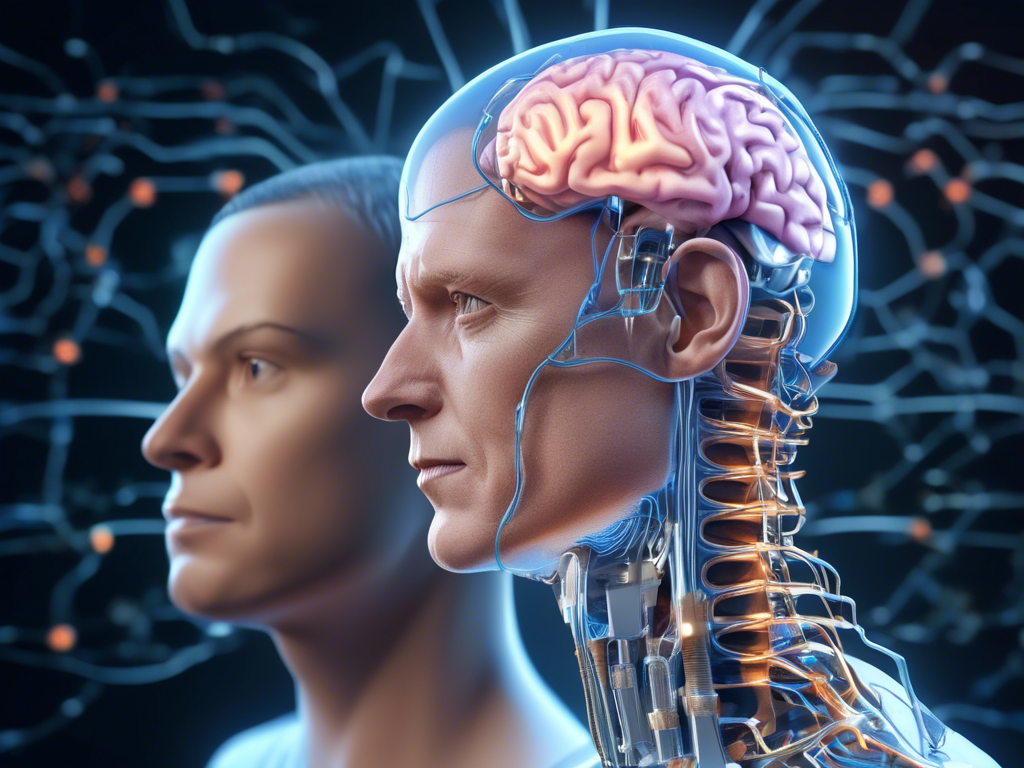
Exploring Neuralink’s Revolutionary Brain-Chip Device
Neuralink, a brain-chip company founded by Elon Musk, is embarking on a groundbreaking study to evaluate its innovative device designed to assist paralyzed individuals in using digital devices through the power of thought alone. This study, which is anticipated to span several years, aims to enroll three patients and could potentially revolutionize the way individuals with spinal cord injuries interact with technology.
The Groundbreaking Neuralink Study
Neuralink’s initiative to enroll patients in its clinical trial has sparked significant interest and discussion within the scientific community and regulatory authorities. Here are some key points about the study:
– The study, which is scheduled to conclude in 2031, aims to enroll patients ranging from 22 to 75 years old with conditions like quadriplegia.
– Eligible patients must have limited mobility for at least a year and a life expectancy of at least 12 months.
– Patients must exhibit minimal to no hand, wrist, and arm movement due to spinal cord injuries or ALS.
Transparency Concerns and FDA Approval
Before Neuralink released details of its clinical trial, the company faced criticism for withholding information about the study, contrary to industry norms. Regulatory bodies like the FDA typically advocate for transparency in clinical trials to bolster public trust and recognize the participants’ contributions. Despite this criticism, the FDA approved Neuralink’s study, emphasizing the importance of sharing study information.
Implanting the Brain-Computer Interface
Neuralink’s study involves surgically implanting a brain-computer interface (BCI) in the brain region responsible for movement intentions. Here are some key details about the implantation process:
– The study uses robotic assistance to precisely position the BCI in the brain.
– Early feasibility studies like this one are crucial for assessing the safety and efficacy of such advanced technologies.
– Neuralink’s first patient, Noland Arbaugh, who is paralyzed from the shoulders down, received the implant in January.
The Impact of Neuralink’s Device
Neuralink’s revolutionary brain-chip device holds immense promise for improving the quality of life for individuals with severe mobility limitations. By enabling paralyzed patients to control digital devices through their thoughts, this technology has the potential to transform how people with spinal cord injuries interact with the world around them.
Hot Take: The Future of Brain-Computer Interfaces
As Neuralink’s study unfolds and more patients benefit from their cutting-edge technology, the realm of brain-computer interfaces is set to witness unprecedented advancements. With the potential to enhance the lives of individuals with mobility impairments, Neuralink’s innovative approach signals a new era in neurotechnology.




 By
By
 By
By
 By
By
 By
By
 By
By